45++ Ph Solution Chart
Ph Solution Chart. 1 mol/l = 10 0 , ph = 0 (acidic) Why is ph important for hydroponics?

Acetate buffer solution ph 4.7. The ph sensitivity of the gel layer. Let us consider the chemical acetic acid having a concentration of 5 molarity.
placard bureau porte coulissante peinture multisupport bricomarche papier peint raye noir et blanc castorama papier peint relief
Red Cabbage pH Indicator Kitchen Chemistry for Kids
Then the ph value of 5m of acetic acid is equal to 2.03 Why is ph important for hydroponics? In chemistry, ph (/ p iː ˈ eɪ tʃ /, historically denoting potential of hydrogen (or power of hydrogen) is a scale used to specify the acidity or basicity of an aqueous solution.acidic solutions (solutions with higher concentrations of h + ions) are measured to have lower ph values than basic or alkaline solutions. The ph scale is a number scale from 0 to 14.

The ph to h + formula that represents this relation is: The ph value is defined, by the sorenson equation, as the negative logarithm of the h+ concentration in a given solution (see table 1). Why is ph important for hydroponics? Acetate buffer solution ph 4.7. 1 mol/l = 10 0 , ph = 0 (acidic)

The ph value is defined, by the sorenson equation, as the negative logarithm of the h+ concentration in a given solution (see table 1). Use this acids and bases chart to find the relative strength of the most common acids and bases. Application of good practices with measurement in aqueous solutions and ph range 1 to 12. Solutions carrying the.
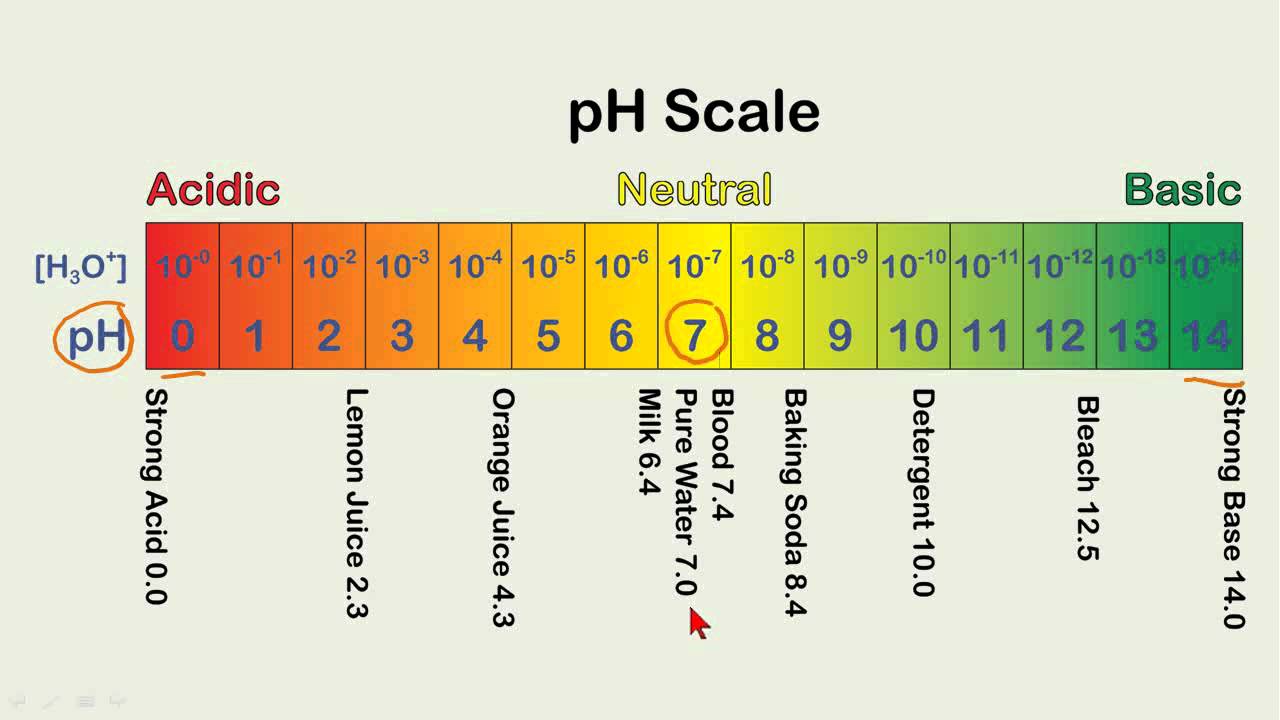
The ph value is defined, by the sorenson equation, as the negative logarithm of the h+ concentration in a given solution (see table 1). A ph scale is a tool for measuring acids and bases. But, you need to know the density of the solution to calculate the mass of solution. It tells us how acidic or alkaline an. The.

The acid and base chart is a reference table designed to make determining the strength of acids and bases simpler. The full form of ph is the potential of hydrogen. Water molecules (h 2 o) can interact with one another to form h 3 o + ions and oh − ions. For an aqueous solution with a temperature of 25°c.

The ph to h + formula that represents this relation is: Students can measure the ph value by using a ph scale. In other words, at a high concentration, e.g. Bases cause universal indicator to change from green toward purple. As you can see from the ph scale above, pure water has a ph value of.
![Calculating pH, pOH, [H+], [H3O+], [OH] of Acids and Calculating pH, pOH, [H+], [H3O+], [OH] of Acids and](https://i.ytimg.com/vi/UiK37I159fc/maxresdefault.jpg)
The paper strip is then compared to a color chart to determine the ph level of the solution being checked. A ph scale is a tool for measuring acids and bases. At 35°c, a neutral solution has ph 6.92. Application of good practices with measurement in aqueous solutions and ph range 1 to 12. Understanding ph > ph indicator ranges;

It commonly ranges between 0 and 14, but can go beyond these values if sufficiently acidic/basic. The ph to h + formula that represents this relation is: The ph scale ranges from 0 to 14 in water. The flip side, of course, is that a strongly basic solution can have 100,000,000,000,000. In chemistry, ph (/ p iː ˈ eɪ tʃ.